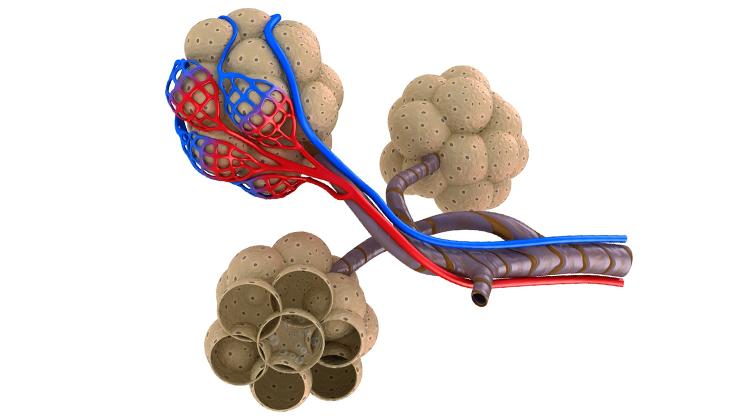
Rice University bioengineer Jordan Miller develops novel biomaterials and 3D bioprinting systems for next-generation tissue engineering applications. These technologies aim to build living tissue architectures that can closely mimic features of native vascular networks within three-dimensional hydrogel scaffolds containing live cells.
New research in the Miller lab, which is supported by a highly competitive Medical Research Grant by the Robert J. Kleberg, Jr. and Helen C. Kleberg Foundation, involves the design, fabrication, and study of sophisticated 3D-printed hydrogel scaffolds that contain perfusable architectures designed to mimic the lung.
In presenting his research discoveries and progress toward a path of clinical translation, Miller explains his focus to overcome bioengineering challenges that link complex cellular microenvironments to the form and function of the three-dimensional scaffold platforms engineered in his lab for tissue fabrication.

“Organs like the lung are pervaded by incredibly elegant, yet extraordinarily complex vascular networks and airways. Bioprinting multiscale living tissues involves more than culturing a few cells within a three-dimensional gel. We must comprehensively consider the cell types to use, optimize their spatial arrangement, provide vascular conduits for fluid convection, and build bioreactors to facilitate the tissue’s maturation,” said Miller, an assistant professor of bioengineering.
By further developing low-cost, high-precision, and open-source 3D printing technologies toward bioprinting, Miller will use the new grant to develop stereolithographic bioprinting strategies to engineer vascularized tissue networks within synthetic biomimetic scaffolds, which they also synthesize in the lab. The platform technology will allow the lab to quantitatively study and optimize vascular oxygen transport, test the compatibility of biomaterials with lung-specific cell types, and explore advanced topological designs that promote large-scale vessel branching and extension.
We must comprehensively consider the cell types to use, optimize their spatial arrangement, provide vascular conduits for fluid convection, and build bioreactors to facilitate the tissue’s maturation
“By introducing unique vessel topologies within advanced hydrogel scaffolds, we are developing a unique system to investigate and test biomechanical influences in lung tissue, such as the cyclic stretching forces imposed during normal breathing,” Miller added.
Native microvasculature is highly variable between organs and between people, yet follows conserved architectural rules that promote nutrient and oxygen transport. The Kleberg Foundation grant will enable Miller’s creation of a family of tunable network architectures, or topographies, through a deterministic computational growth algorithm.
Although the ability to reproduce replacement human organs lies in the future, successful completion of the project will advance our understanding of the unique interplay between structure and function within lung tissue, and lay a transformative foundation for pre-clinical testing.
The Robert J. Kleberg, Jr. and Helen C. Kleberg Foundation was established in 1950 in Kingsville, Texas, to support the philanthropic interests of its founders. Now based in San Antonio, it has a mission to improve the world through the advancement of knowledge in medical and basic science research, conservation, community services and the arts. To date, over $302M in grants has been awarded with a major focus on medical and basic science research.